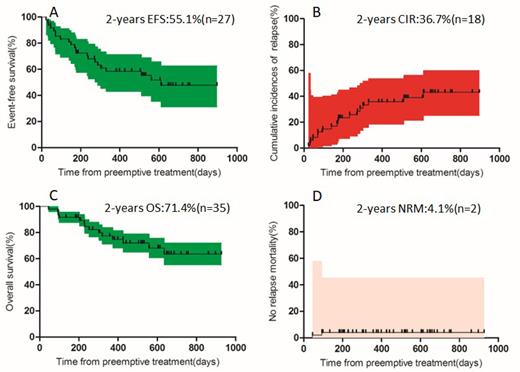
Objective : this study investigated the efficacy and safety of azacitidine and interferon α preemptive treatment in the post-transplant in patients with AML/MDS. Methods: between Octorber 1, 2019 and July 31, 2022, a total of 49 patients aged 17 to 62 years were enrolled into this prospective clinical study of post-transplant azacitidine combined with interferon α preemptive treatment. Patients with minimal residual disease (MRD) positive and / or mixed chimerism were scheduled to receive azacitidine, given as 32 mg/m 2 per day subcutaneously for 5 days, and interferon α 3 million units/m 2 per day from 8th day and continued for 28th day for 1 cycle. Results: patients who acquired treatment response were 34 (69.4%), of which turned MRD-negative and obtained complete chimerism were 22 (44.9%) and 5 (10.2%) at 1 cycle, respectively. Median follow-up time after preemptive intervention was 440(range, 45 to 927) days. Cumulative incidences of relapse (CIR) and no relapse mortality (NRM) were 36.7% and 4.1%, respectively. Probabilities of event-free survival (EFS) and overall survival (OS) were 55.1% and 71.4%, respectively. The most common adverse events were graft-versus-host disease (GVHD) (38.8%), liver toxicity (46.9%) and neutropenia (28.6%). Conclusion: these data support the notion that azacitidine combined with interferon α is better used as the preemptive therapy against relapse tendency for patients after allo-HSCT performed for AML/MDS. Side effects were mainly GVHD, reversible cytopenia and hepatic toxicity. This prospective trial in the post-transplant setting was feasible and safe but challenging. This trial was registered at www.clinicaltrials.gov as#NCT04078399.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal